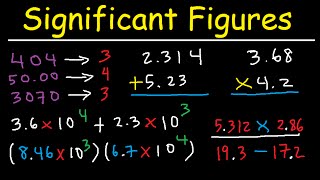
Significant Figures Rules Addition Subtraction Multiplication Division Examples
It shows you how to round to the correct decimal. These rules also differ depending on whether you are combining numbers in an addition or subtraction or performing multiplication or division.

Identifying Significant Figures Worksheet Chemistry Worksheets Worksheets Practices Worksheets
Add up the number of significant figures to the right of.

Significant figures rules addition subtraction multiplication division examples. Any zeros between two significant digits are significant. 9 5 32 F C In this equation 32 9 and 5 are exact numbers see rule 1. Rules for Significant Figures.
4231351 7741 4231. 2378-12 1178 in approach 1 by rounding this down to 2 significant digits 12 you are reducing the accuracy of your calculation before you finish. The final answer limited to four significant figures is 4094.
804003 has six significant figures. Addition rules and subtraction rules for significant figures. Your final answer may have no more significant figures to the right of the decimal than the LEAST number of significant figures in any number in.
For addition and subtraction for example if the first number is a 01 and the second is b 0001 and the numbers are independent of each other and normally distributed the absolute error of the sum is sqrt012 00012 That comes out to just about 01. In the example below this would be 111 this is the least precise quantity. An example is as follows.
Lets start by talking about how addition and subtraction work when dealing with significant figures. But since the length and width only have two significant figures the area will only have two significant figures. The first digit dropped is 1 so we do not round up.
For addition and subtraction use the following rules. Zeros that do nothing but set the decimal point are not significant. If the length was L 32 meters and the width was W 28 meters then the area would be A L x W 32 m 28 m 896 m 2.
This chemistry and physics video tutorial provides an introduction basic overview on significant figures. Rules for counting significant figures are summarized below. For multiplication or division the rule is to count the number of significant figures in each number being multiplied or divided and then limit the significant figures in the answer to the lowest count.
What is Significant Figure and their rules and Addition Subtraction Multiplication and Division Significant Figure kiya he or Significant Figure ke rules. Convert 73F to K Kelvin STEP 1. Add or subtract in the normal fashion 3.
Rules for multiplicationdivision problems. For quantities created from measured quantities via multiplication and division the calculated result should have as many significant figures as the least number of significant figures among the measured quantities used in the calculation. C C 228 23 9 5 41 9 5 73 32 Note that in this equation 73 contains 2 significant figures and the.
A final zero or trailing zeros in the decimal portion ONLY are significant. The rules for determining the number of significant figures are. Count the number of significant figures in the decimal portion ONLY of each number in the problem 2.
It is important to be aware of significant figures when performing mathematical operations. To use your example when calculating the subtraction part. Convert 73F to C.
Addition and subtraction are performed similarly to normal addition and subtraction. Calculations Involving MultiplicationDivision and AdditionSubtraction. For example dividing 125 by 307 on a calculator gives 04071661238 to an infinite number of.
7939 626 111 25299 this is what your calculator spits out In this case your final answer is limited to one sig fig to the right of the decimal or 253 rounded up. Rules for determining significant figures. 1234 20 2.
Multiplication and division. You leave the rounding til the very end that is when you report your answer only so many digits are significant. Significant Figures With Both Addition And Multiplication Operations Example.
32684 has five significant figures. All non zero digits are significant. For example 1234 2 2468 2.
Non-zero digits are always significant. Use significant figures correctly in mathematical operations. Zeros withina number are always significant.
To determine the number of significant figures in a number use the following 3 rules. All zeroes occurring in between two non zero digits are significant. Both 4308 and 4005 contain foursignificant figures.
Addition Subtraction and Significant Figures. For example the addition of the following number gives an answer with 4 figures. 5 00 or 632 000 the zeros are significant.
The result of an addition or subtraction problem should always contain the same number of decimal places as the input value with the fewest number of decimal places. On the other hand if both have a uncertainty of 01 the uncertainty of the sum is actually a bit higher approximately 014. 4 68 25.
You therefore report the area as A 90 m 2.

Covers How To Determine The Significant Figures In Problems Containing A Combination Of Addition X2f Subtraction Scientific Notation Homeschool Math Math Help

03 Significant Figures Rules Sig Fig Rules For Calculations In Chemistry Physics Youtube Homeschool Math Physics Scientific Notation

Science And Measurement Chemistry Is My Jam Chemistry Lessons Chemistry Gcse Math

Includes Concept Summary Handouts For Students 20 Question Practice Quiz With Full Answer Key 50 Task Cards Task Cards High School Science Math Courses

Science And Measurement Chemistry Is My Jam Chemistry Classroom Chemistry Science

Physics The Significant Digits Drill Software Consists Of A Set Of Tutorials And Drills The Tutorials Use Step By St Teaching Science Basic Concepts Science

Science And Measurement Chemistry Is My Jam Chemistry Notes Chemistry Chemistry Classroom

I Pinimg Com Originals A3 8c 96 A38c963eee9945f

Multiplying And Dividing With Significant Digits Worksheets Scientific Notation Worksheet Chemistry Worksheets Scientific Notation

No Title Provided Teaching Chemistry Chemistry Classroom College Chemistry

Converting To And From Scientific Notation A Plus Topper Scientific Notation Scientific Notation Anchor Chart Notations

Decimal Operations Anchor Chart Colour Coded Step By Step With Examples Adding Decmimals Subtracting Math Anchor Charts Fifth Grade Math Sixth Grade Math

Integer Rule Cheat Sheet On Tpt Integer Rules Teaching Integers

Science And Measurement Chemistry Is My Jam Scientific Notation Teaching Science Chemistry Classroom

Mathematics Pret Homeworks Number Learn Squared Subtraction Mathematics

Posters Rules For Significant Figures High School Science And Math High School Science High School Math Science

Scientific Notation Notes And Practice Scientific Notation Scientific Notation Notes Scientific Notation Worksheet